Abstract
OBJECTIVE: POEMS syndrome is a rare plasma cell dyscrasia and there is currently no standard treatment. Current treatments include melphalan, autologous stem cell transplantation (ASCT) and novel regimens. However, the efficacy of these treatments has not previously been retrospectively compared in large cohorts. Therefore, this study aims to compare the efficacy and survival of three treatment regimens in patients with POEMS syndrome in our 18-year cohort.
METHODS: We retrospectively analyzed the clinical records of 347 patients with newly diagnosed POEMS syndrome who were diagnosed and treated in our hospital from January 2000 to December 2017 with complete treatment and follow-up data. Patients were divided into three groups according to the initial first-line treatment regimen: melphalan + dexamethasone (MDex, N = 79) for 9 months, autologous stem cell transplantation (ASCT, N = 165), or lenalidomide + dexamethasone for 1 year (LDex, N= 103). Hematologic complete remission rates (CRH), vascular endothelial growth factor (VEGF) complete remission rates (CRV), and neurological remission rates (RN) were compared for three regimens, as well as progression-free survival (PFS) and overall survival (OS). Hematologic complete remission was negative IFE and no FLC in serum or urine. The VEGF complete remission was normalized VEGF (<600 pg/ml). The neurological response was defined as the reduction of ONLS by more than 1 point. Remission rates were compared by Pearson ӽ 2 test or Fisher's exact test. Survival curves were plotted by the Kaplan-Meier method and tested using a log-rank test. Subgroup analyses were based on risk stratification and heterogeneous baseline characteristics between treatment groups.
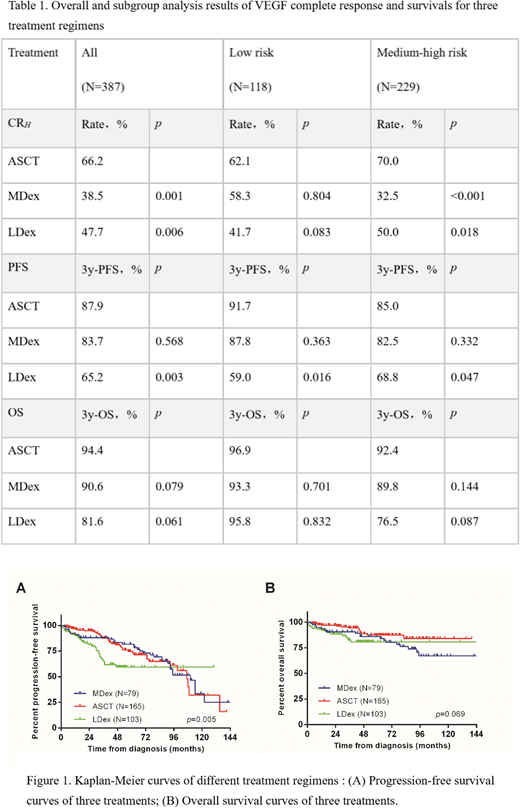
RESULTS: Patients in the ASCT group achieved significantly higher rates of CRV (66.2%), which were superior to the MDex group (38.5%, p = 0.001) and the LDex group (47.7%, p = 0.006). There were no significant differences in CRH and RN between the three groups: CRH (MDex 37.7% vs ASCT 49.7% vs LDex 47.5%, p = 0.244) and RN (MDex 100% vs ASCT 91.% vs LDex 93.8%, p = 0.234). However, the proportion of low risk patients in the ASCT group was significantly higher than that in the MDex and LDex groups (44.2% vs 22.8% & 26.2%, p=0.001). Therefore, we performed a subgroup analysis. Subgroup analysis showed that the CRV in the ASCT group was similar to that in MDex and LDex groups in the low-risk group (p = 0.222), but there was a significant advantage in the middle- and high-risk patients (p = 0.001). After a median follow-up of 45 months, a total of 49 deaths occurred and 50 patients developed disease progressions but remained alive. The 3-year PFS was 83.7% for MDex group, 87.9% for ASCT group, and 65.2% for LDex groups. The 3-year OS was as follows: MDex group 90.6%, ASCT group 94.4%, and LDex group 81.6%. The ASCT group achieved a better PFS than the LDex group (p = 0.003), while the three groups had no significant differences in 3-year OS (p = 0.069).
CONCLUSION: All three treatment regimens have achieved good efficacy and survival in the treatment of POEMS syndrome. Compared with melphalan and lenalidomide-based regimens, patients at medium to high risk may benefit more from autologous transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal